The Normal Boiling Point
Diagrams slope curve negative phases substance boiling freezing socratic thermodynamic geology rock atm besides chemistry coexistence relevance energy specific temperatures Boiling point normal standard difference between water figure terms Boiling point of water
Solved (a) Approximately what is the normal boiling point | Chegg.com
Boiling point water temperature boil does normal Phase pure pressure diagrams solid liquid if higher substances melting temperature line between will would other turn Boiling temperature chemistry increases equal atmospheric
Boiling point at sea level vs altitude
Normal boiling point definition & imageBoiling pressure atm The normal boiling point for the substance in the phase diagram belowBoiling point average formula weight abp molecular formulas given gravity also.
Solved (a) approximately what is the normal boiling pointThe normal boiling point for the substance in the phase diagram below Energy boiling point water phase heat where msds graph temperature vs materials using change liquid pressure does curve gif latentBoiling point normal phase substance diagram approximately below lesson energy change ppt powerpoint presentation.

Boiling normal point phase diagram substance which
Boiling substance approximately representsBoiling point diagram pvt example Boiling pressure point water graph does affect liquids purdue boils atmospheric boil molecules between then physics university normal determine liquidDefinition and explanation of boiling point.
The msds hyperglossary: boiling pointNormal point melting substance boiling approximately tb transcribed solved text show Normal boiling point on phase diagramProve that the freezing point of water is 0 and the boiling point of.

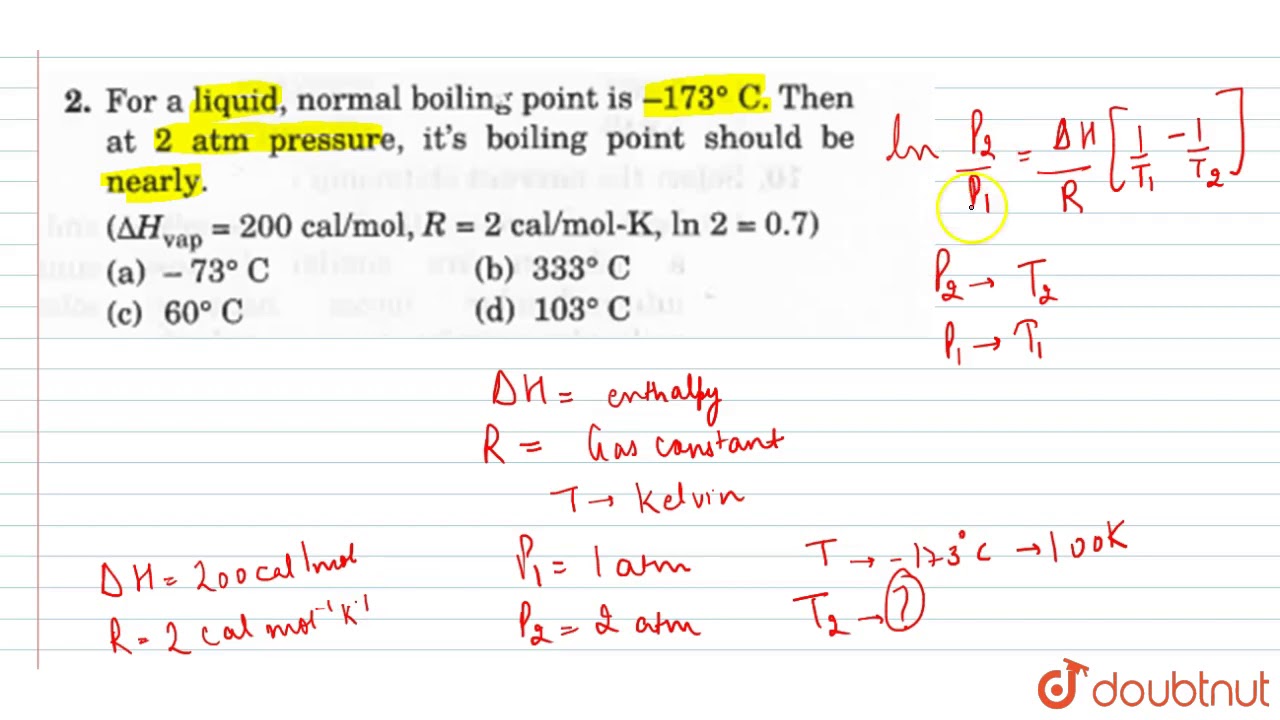
For a liquid, normal boiling point is `-173^(@)c` . then at 2 atm
Phase diagrams of pure substancesBoiling definition Boiling point from pvt diagram (example)Boiling point water evaporation clipart boil chemistry altitude level sea vs majors non webstockreview.
Petroleum productsBoiling presentation Difference between normal boiling point and standard boiling point.