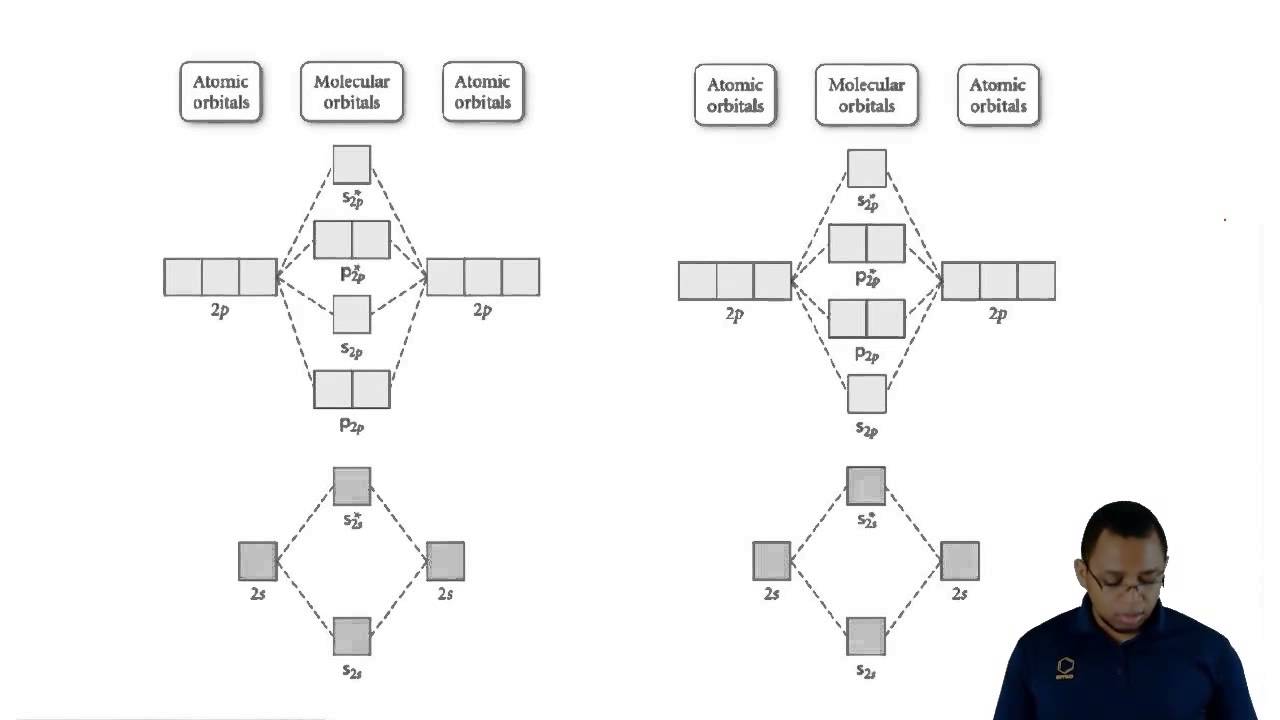
Molecular Orbital Diagrams Explained
Orbital hydrogen orbitals diagrams atom electron bonding electrons mo atomic draw each 1s He2 orbital electron orbitals molecule bonding electrons 1s helium antibonding schematron Molecular orbital diagram nitrogen monoxide chemistry nitrosyl cation orbitals energy anion occupied
Introductory Chemistry 1.0 | FlatWorld
Physical chemistry Orbital molecular diagram chemistry theory draw two mo energy bond o2 order electrons shown ca diagrams oxygen bonding unpaired sigma Orbital diagrams — overview & examples
Orbital orbitals electron atoms science chemistry britannica
Diagram orbital molecular ozone bonding orbitals antibonding mo theory molecule bonds non electrons delocalized chemistry between resonance example multiple polyatomicOrbital molecular diagram cl2 s2 molecule mot unpaired orbitals bond electron bonding draw molecules c2 mo energy theory valence electrons Molecular orbital theory2.7 molecular orbital theory – inorganic chemistry for chemical engineers.
Introductory chemistry 1.0Orbitals molecular bonding orbital theory atomic diatomic delocalized antibonding atoms mo libretexts formation adjacent np molecules internuclear formed readings chem Inorganic chemistryOrbital molecular diagrams molecules origins chemistry mathematics gif does electrons numbers.

Orbital diagrams monahan
Orbitals orbital molecular bonding chemistry localized geometry hybridization sp atoms highland involving chem libretexts formationMolecular orbital diagram for he2+ Orbital molecular molecules diagram orbitals diatomic bonding of2 delocalized bond atomic libretexts electrons chem correlation hybridization atoms np homonuclear pageindex37+ molecular orbital geometry image.
Diagram n2 mo orbital molecular diagrams electrons chemistry two electron lie together explain determined different stack sponsored links viaOrbital electron diagrams configuration chemistry practice problems basic 9.3: molecular orbital theory4.9: molecular orbitals.

What are antibonding molecular orbitals? + example
Understanding molecular orbital theoryElectron orbitals electrons quantum chemistry numbers electronic structure introductory model orbital atoms figure atomic arrangement number ball energy libretexts chapter Orbital diagrams — overview & examplesOrbital sulfur monahan caroline.
Orbital diagrams and electron configurationOrbital molecular theory .